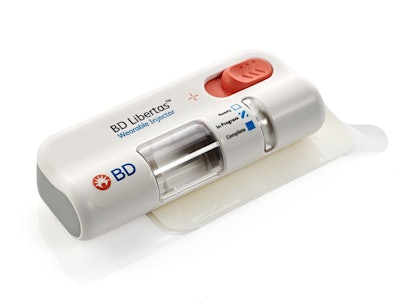
BD (Becton, Dickinson and Company) announces the first pharma-sponsored combination product clinical trial using the BD Libertas™ Wearable Injector for subcutaneous delivery of complex biologics.
The selection of BD Libertas™ Wearable Injector for this pharma-sponsored trial follows successful outcomes from more than 50 BD-conducted pre-clinical and clinical studies, including a device clinical study demonstrating excellent performance with 100% of study participants stating they would likely use the BD Libertas™ Wearable Injector if prescribed.
The pharma-sponsored combination product clinical trial represents a significant advancement in accelerating innovation in drug-device combination products that provide greater flexibility for patients, including potential conversion from infused medications that require patients to travel to a hospital or clinic to more convenient patient care in various settings, including self-injection at home.
"This trial demonstrates BD's commitment to helping pharma companies by advancing large-volume injection science, ensuring therapies are accessible and patient friendly by offering more efficient and convenient options for biologics,” says Patrick Jeukenne, worldwide president of BD Pharmaceutical Systems. "BD’s enhanced testing capabilities acquired through ZebraSci and the proven capabilities of the BD Libertas™ Wearable Injector technology further position BD as an innovative leader in drug delivery.”
The BD Libertas™ Wearable Injector is an innovative, prefilled, patient ready-to-use drug delivery system designed to enable delivery of complex biologics via subcutaneous injection. The biologics market is expected to grow to more than $670 billioniv by 2030 and for pharmaceutical companies developing these complex drugs, the BD Libertas™ Wearable Injector offers a customizable, patient-centric solution. The BD Libertas™ Wearable Injector:
- Supports delivery of high-viscosity biologics (up to 50 centipoise), enabling a wide range of subcutaneous therapies.
- Is offered in 2 to 5 mL and 5 to 10 mL configurations providing flexibility for diverse therapeutic requirements.
- Features a fully mechanical, patient ready-to-use design with a simple “peel, stick and click” mechanism, requiring no end-user filling or assembly.
BD’s ongoing validations of fill-finish and final assembly processes with multiple Contract Manufacturing Organizations (CMOs) enable the company to support pharmaceutical partners from development through commercial-scale production. For more information about how BD Libertas™ Wearable Injector is enabling biologic therapy delivery, visit www.bd.com/libertas.