This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
IL Group, a specialist in multifunctional labeling solutions for the pharmaceuticals and healthcare sectors, has announced the results of a recent study validating the effectiveness of its Vial Protect Pack (VPP) shrink tack labels in preventing the external contamination of vials.
The study evaluated 150 vials containing the cytotoxic drugs doxorubicin, paclitaxel, and gemcitabine. All vials were wrapped using IL Group’s proprietary Vial Protect Pack labels. Results showed no measurable contamination on the external surfaces of any VPP-wrapped vials, with drug concentrations consistently below detection thresholds in every sample.
External contamination of hazardous drug vials presents a well-documented occupational risk to healthcare workers such as pharmacists, nurses, and pharmacy technicians. Studies have shown that over half of commercial cytotoxic vials may carry trace contamination on their outer surfaces—even when compounded in isolators or biological safety cabinets. By reducing external contamination to undetectable levels, Vial Protect Pack provides a layer of defense, helping to mitigate exposure risks during the drug preparation process and throughout the supply chain.
To perform the study, wipe sampling was performed using Cyto Wipe Kits. All vials paired with IL Group’s Vial Protect Pack solutions, including their signature protective bottoms and flip-off caps, were wiped using a single tissue. After sampling, the tissue with placed in a container with 0.1% of formic acid added. Samples were then extracted and analyzed using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) to detect even trace levels of the tested drugs.
The study conclusively found no measurable contamination on the external surfaces of any of the 150 VPP-wrapped vials. The findings demonstrate that Vial Protect Pack technology reduces external drug residue to undetectable levels, supporting cleaner, safer pharmaceutical handling processes.
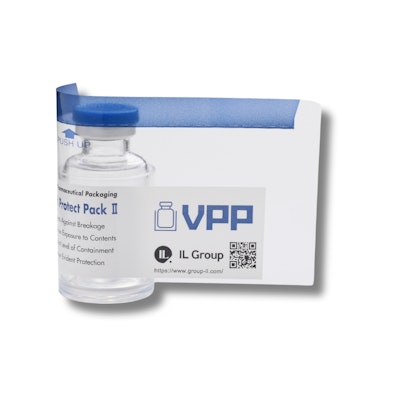
IL Group’s Vial Protect Pack (VPP) series is designed to provide added protection for fragile or high-leverage glass vials, as well as outer surface contamination of cytotoxic medicine. Comprising a glass vial, plastic protector, and Shrink Tack Label, the system delivers both reliable safeguarding and tamper evidence. The label’s adhesive securely bonds the plastic component to the primary container, eliminating any risk of unintended separation and enhancing protection throughout the supply chain.
The VPP series is available in two configurations: Vial Protect Pack I (VPP I), which features a bottom plastic plate attached to the vial with the Shrink Tack Label for enhanced protection; and Vial Protect Pack II (VPP II), which essentially provides a “container for a container.” The solution combines a thick, tall plastic cup that cradles the primary vial with a shrink-tack label offering reliable tamper evidence. The plastic cup and the primary container are held in place with the label’s adhesive, eliminating any chance for their unintended separation. Both versions incorporate IL Group’s proprietary Shrink Tack Label, a heat-shrinkable PET film with pressure-sensitive adhesive for a secure bond and superior protection.
“These findings reinforce the importance of Vial Protect Pack as a preventive solution that enhances worker safety and protects the hospital environment,” says Hideo Yonenaga, Group Manager at IL Pharma Packaging. “We’re proud to offer pharmaceutical manufacturers and healthcare providers a technology that meaningfully reduces risk in the handling of hazardous drugs.”